HTML Preview Executive Visit Agenda page number 1.
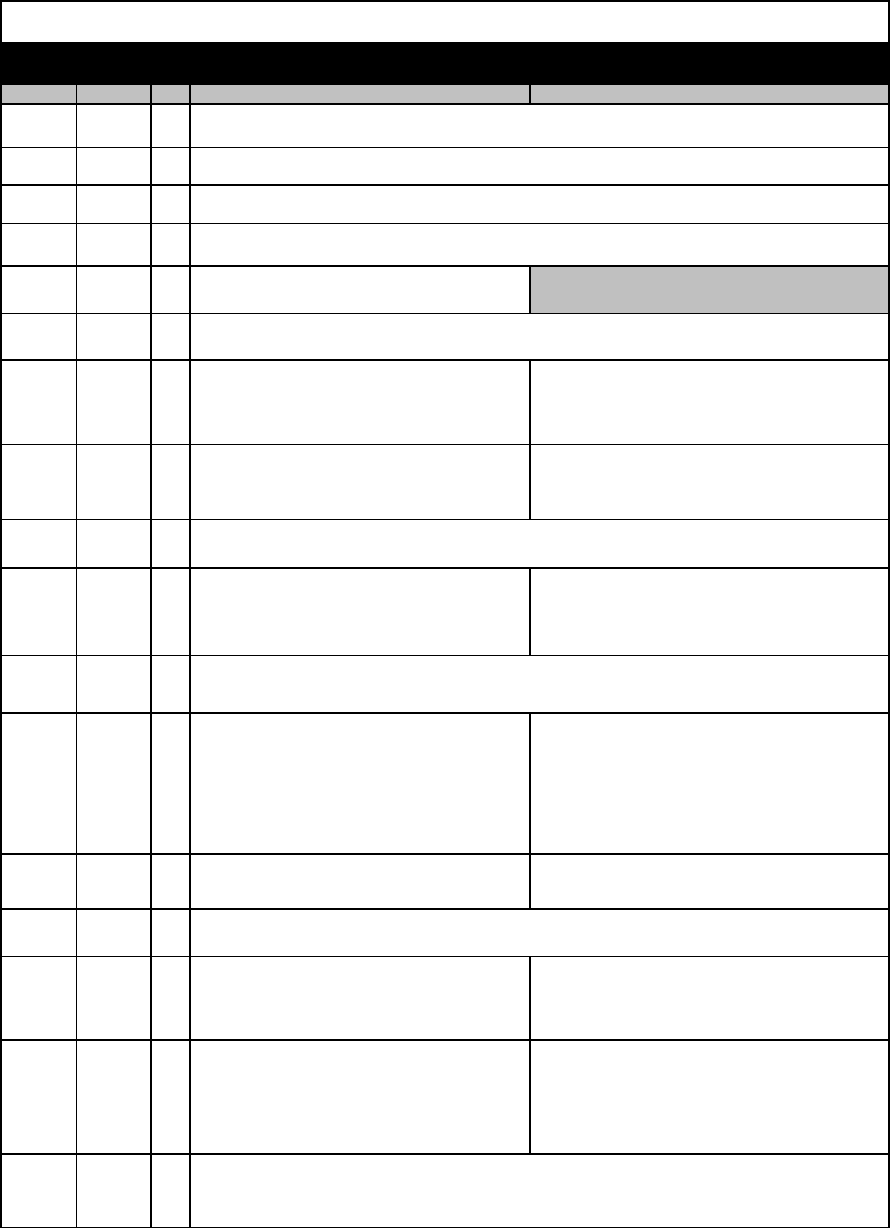
Draft Site Visit Agenda - University of Southern California - 2006-078 4/23/2015
Start End
Min
Team A - HSC Team B - Start at HSC, End at UPC
8:00 AM 8:15 AM 15
8:15 AM 8:30 AM 15
8:30 AM 9:00 AM 30
9:00 AM 9:15 AM 15
9:15 AM 9:45 AM 30 Records Review Travel to UPC
9:45 AM 10:45 AM 60
10:45 AM 11:30 AM 45
Researchers (Biomedical/Clinical):
Mikhaela Cielo;
Kevin Kelly; Wendy Gilmore
Researchers (Social/Behavioral/Educational): Alice
Cepeda; Kyle Konis; Jeremy Goldbach
11:30 AM 12:00 PM 30
Person in Charge of Managing Conflict of
Interest, HSC: Frances Richmond, PhD
Executive Director, USC Stevens Center for
Innovation - Person in charge of Technology
Transfer and Patents: Jennifer Dyer
12:00 PM 12:05 PM 5
12:05 PM 12:50 PM 45
HSIRB (S) (Biomedical/Clinical - Initial Review) -
HSIRB 1: Keith Lewis, MD; Luanda Grazette, MD;
HSIRB 2: Michael Levine, MD; Alice Stek, MD
UPIRB (S): Carol Prescott, PhD; Lisa Hope, PsyD; Ann
Marie Yamada, PhD
12:50 PM 2:05 PM 75
2:05 PM 2:50 PM 45 Records Review & Lunch
Legal Counsels to the IRB - University Counsel:
Stephen Yamaguchi, JD; Associate Senior Vice
President, Compliance: Laura LaCorte, JD; Research
Compliance Director; Person who Conducts Audits
of the HRPP (reports to Ms. LaCorte):
Daniel Shapiro,
JD
2:50 PM 3:20 PM 30
Representative from Research Pharmacy:
Kim Le,
Pharm D
Chair, UPIRB: Julie Slayton, PhD
3:20 PM 3:25 PM 5
3:25 PM 3:55 PM 30 Director of Clinical Trials Office: Soheil Jadali IRB Director, UPIRB: Kristin Craun, MPH
3:55 PM 4:40 PM 45
Chair, HSIRB: Darcy Spicer, MD; Vice-Chairs,
HSIRB: Deirdre Anglin, MD, MPH; Robert Larsen,
MD; Linda Sher, MD
Staff for Researchers
(Social/Behavioral/Educational): Suzanne Wenzel;
Jeremy Goldbach; Ferol Mennen
4:40 PM 5:00 PM 20
AAHRPP Site Visit:
University of Southern California
Wednesday
May 6, 2015
Introductions
Electronic Records Tutorial
Executive Session
Records Review & Lunch
Program Overview
Organizational Questions
Records Review
Executive Session
End of Day Session with Application Contact (Team B Phone In)