HTML Preview Clinical Trial Budget page number 1.
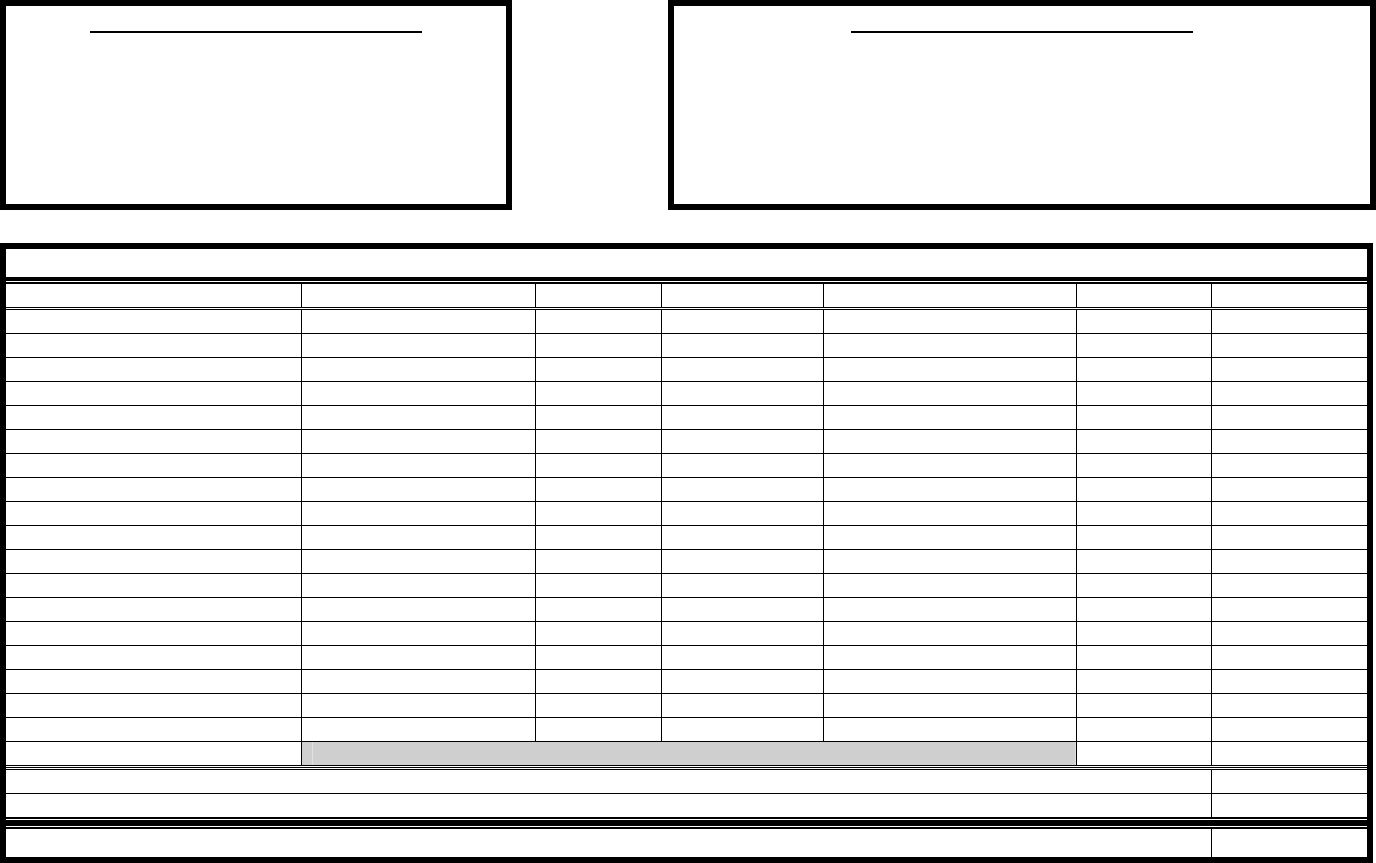
Clinical Trials Budget Worksheet (internal document)
Study Name: ____________________________________ Sponsor: ______________________________
Investigator: ____________________________________ Project Period Begins: ______________________
Study Coordinator: ______________________________
INSTITUTIONAL START-UP INVESTIGATOR START-UP *
Non-refundable, one time fees Time / Effort for Protocol Assessment
IRB Fee: ____________ Protocol Review (PI + Coordinator): ____________
Pharmacy Fee: ____________ Site Selection Time w / Sponsor: ____________
Regulatory Asst. Fee: ____________ Investigator Meeting: ____________
In-Service Staff: ____________
Subtotal: $__________
*This is separate from an advance
Subtotal: $__________
N.B. – Advertising Costs can be negotiated into the contract (usually given as a total “up to…” identified $$ amount) – paid by invoice
PER SUBJECT STUDY CHARGES
STUDY ACTIVITIES PERSONNEL (PI/RN) EST. HRS FREQUENCY TOTAL HRS. / PATIENT FEE SUBTOTAL
Physical Exam/Medical History
Consent
Screening
Nursing assessment / Vital Signs
Phlebotomy
Specimen prep & Shipping
Review of meds/protocol
CRF Completion
Queries/Monitor Visits
IRB Correspondence
SAE Forms
Other study specific:
Ex: CXR
ECG
Bone Age
Per Patient Pharmacy Fees
Calculated by Investigational Pharmacist
SUBTOTAL
Institutional Overhead (26%)
TOTAL
Will record storage need to be off-site? Negotiate one-time charge if appropriate